Corrosion and Its Prevention
Corrosion and Its Prevention: Overview
This Topic covers sub-topics such as Galvanization, Relative Humidity, Prevention of Corrosion, Corrosion of Metals, Factors Affecting Corrosion, Sacrificial Protection, Barrier Protection and, Mechanism of Rusting of Iron
Important Questions on Corrosion and Its Prevention
Volatile oxidation corrosion product of a metal is,
Lower the pH, corrosion is :
The apparatus shown was set up with volume of air in the tube. The volume of gas in the tube was measured at intervals for six days.
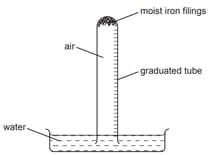
Which graph best represents how the volume of gas changes with time?
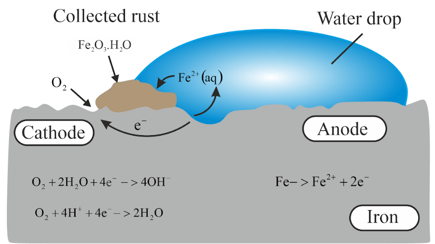
Observe the following picture and write down the chemical reaction with explanation.
Using the values of and predict which one is better for coating the surface of iron to prevent rust and why?
Given :
.
Both aluminium and iron combine slowly with oxygen at room temperature. Why is this reaction a problem for iron but not for aluminium?
Three iron sheets have been coated separately with three metals ( and ) whose standard electrode potentials are given below:
| Metal | Iron | |||
Identify in which case rusting will take place faster when coating is damaged.
Write short notes on Corrosion.
State reason, rusting of iron is said to be an electrochemical phenomenon.
Corrosion is essentially an electrochemical phenomenon. Explain the reactions occurring during corrosion of iron kept in an open atmosphere.
Describe some of the methods used by which the corrosion may be prevented.
What do you understand by the term corrosion ? Describe the theory of corrosion.
Iron does not rust even if zinc coating is broken in a galvanised iron pipe, but rusting occurs much faster if the tin coating over iron is broken. Explain.
What is electrochemical theory of rusting? Explain.
We can use aluminium in place of zinc for cathodic protection of rusting. Comment.
Rusting of iron is quicker in saline water than in ordinary water. Explain.
Describe any two of the techniques used for preventing corrosion of metals.
What is corrosion? Explain any four factors affecting corrosion.
Give a brief account of corrosion and its mechanism.
Explain 'Iron is galvanised for protecting it from rusting'.
